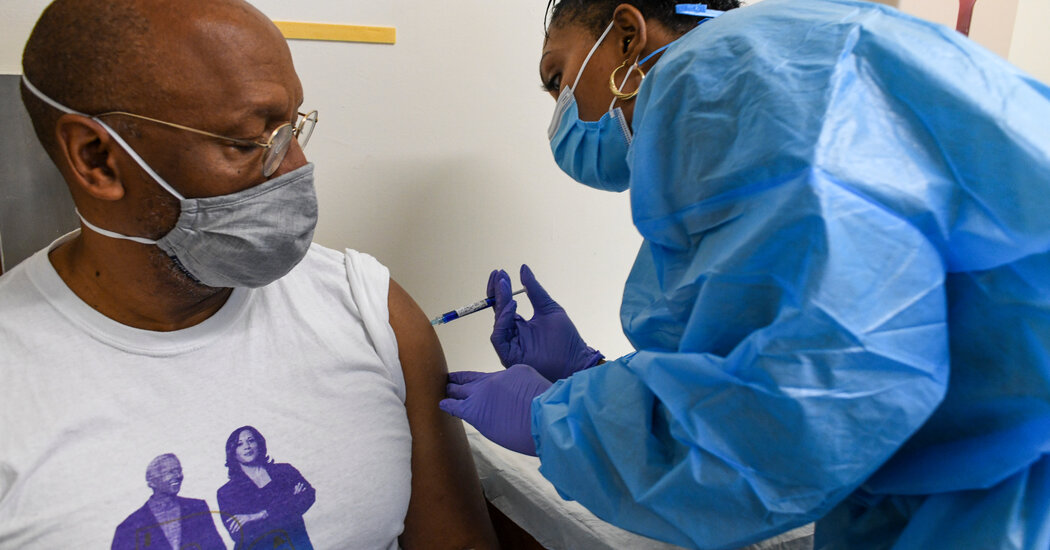
“This is really worrying,” said Dr. Peter Hotez, vaccine expert at Baylor College of Medicine and inventor of a coronavirus vaccine. “We need to vaccinate the American people by late spring or early summer to prevent the South African and British variants from adopting.”
Drug manufacturers could update their vaccines and offer new ones on a regular basis, similar to the flu vaccine.
Covid19 vaccinations>
Answers to your vaccine questions
Am I eligible for the Covid vaccine in my state?
Currently more than 150 million people – almost half of the population – can be vaccinated. But each state makes the final decision on who goes first. The country’s 21 million healthcare workers and three million long-term care residents were the first to qualify. In mid-January, federal officials asked all states to open eligibility to anyone over the age of 65 and adults of any age with medical conditions that are at high risk of becoming seriously ill or dying of Covid-19. Adults in the general population are at the end of the line. If federal and state health authorities can remove bottlenecks in the distribution of vaccines, everyone over the age of 16 is eligible as early as spring or early summer. The vaccine has not been approved in children, although studies are ongoing. It can take months before a vaccine is available to anyone under the age of 16. For the latest information on vaccination guidelines in your area, see your state health website
Is the Vaccine Free?
You shouldn’t have to pay anything out of pocket to get the vaccine, despite being asked for insurance information. If you don’t have insurance, you should still get the vaccine for free. Congress passed law this spring banning insurers from applying cost-sharing such as a co-payment or deductible. It consisted of additional safeguards prohibiting pharmacies, doctors, and hospitals from charging patients, including uninsured patients. Even so, health experts fear that patients will end up in loopholes that make them prone to surprise bills. This could be the case for people who are charged a doctor’s visit fee with their vaccine or for Americans who have certain types of health insurance that are not covered by the new regulations. If you received your vaccine from a doctor’s office or emergency clinic, talk to them about possible hidden costs. To make sure you don’t get a surprise invoice, it is best to get your vaccine at a Department of Health vaccination center or local pharmacy as soon as the shots become more widely available.
Can I choose which vaccine to get?How long does the vaccine last? Do I need another next year?
That is to be determined. It is possible that Covid-19 vaccinations will become an annual event just like the flu vaccination. Or the vaccine may last longer than a year. We’ll have to wait and see how durable the protection from the vaccines is. To determine this, researchers will track down vaccinated people to look for “breakthrough cases” – those people who get Covid-19 despite being vaccinated. This is a sign of a weakening of protection and gives researchers an indication of how long the vaccine will last. They will also monitor the levels of antibodies and T cells in the blood of people who have been vaccinated to see if and when a booster shot might be needed. It is conceivable that people might need boosters every few months, once a year, or just every few years. It’s just a matter of waiting for the data.
Does my employer need vaccinations?Where can I find out more?
“This virus is throwing us curve balls every day. I think we just need to be prepared and realize that the first generation of vaccines may need to be updated,” said Dr. Jesse L. Goodman, professor of medicine and infectious diseases medicine at Georgetown University.
The Novavax study in the UK tested how many volunteers developed symptoms of Covid-19 a week after receiving a second dose. The company said Thursday that its initial analysis found that of 62 participants who got the disease, 56 had received a placebo and 6 had received the vaccine. According to Novavax, the newer, more contagious variant, first identified in the UK, caused about 50 percent of the cases in the study.
If these results were reflected in the larger clinical trial in the United States and Mexico, which involved approximately 16,000 out of 30,000 people, it would equate the vaccine with the Moderna and Pfizer-BioNTech vaccines, which were shown to be about 95 percent are effective.
But the news in South Africa was not that encouraging. Novavax’s smaller study found the vaccine to have an overall effectiveness of 49.4 percent. (The company reported that about 6 percent of the study participants were HIV positive, and for those who weren’t HIV positive, the vaccine had an effectiveness of 60 percent.) The company said the study was conducted from September through September The recording of cases of Covid-19 began midway through this month when the more contagious variant was widespread. According to Novavax, 44 study participants developed Covid-19 and sequenced the genetic lineage of 27 cases. Of these, 25 cases were caused by the more contagious version of the virus.
The company also said that about a third of study participants in South Africa had previously developed Covid-19 after being infected with the original form of the virus, and that their results showed that these previous infections did not protect them from the new variant. The company said its vaccine offered some protection for those who had previously contracted the disease, but did not include that group in its analysis.