“With the UK, we had an additional three months to fix any issues we encountered,” AstraZeneca CEO Pascal Soriot told an Italian newspaper, la Repubblica, this week.
On Friday, the European Union drug regulators approved the AstraZeneca vaccine for all adults, following the precedent set by the UK regulator last month.
Britain could get another vaccine soon.
Novavax, a biotechnology company based in Gaithersburg, Md., Reported Friday that its vaccine was 89.3 percent effective in a large-scale study in the UK. The government has secured 60 million cans made at a facility in north east England. If the UK regulators approve, the vaccine will be dispensed in the second half of 2021.
In total, the UK government has spent at least £ 11.7 billion, or $ 16 billion, developing, manufacturing, buying and administering vaccines.
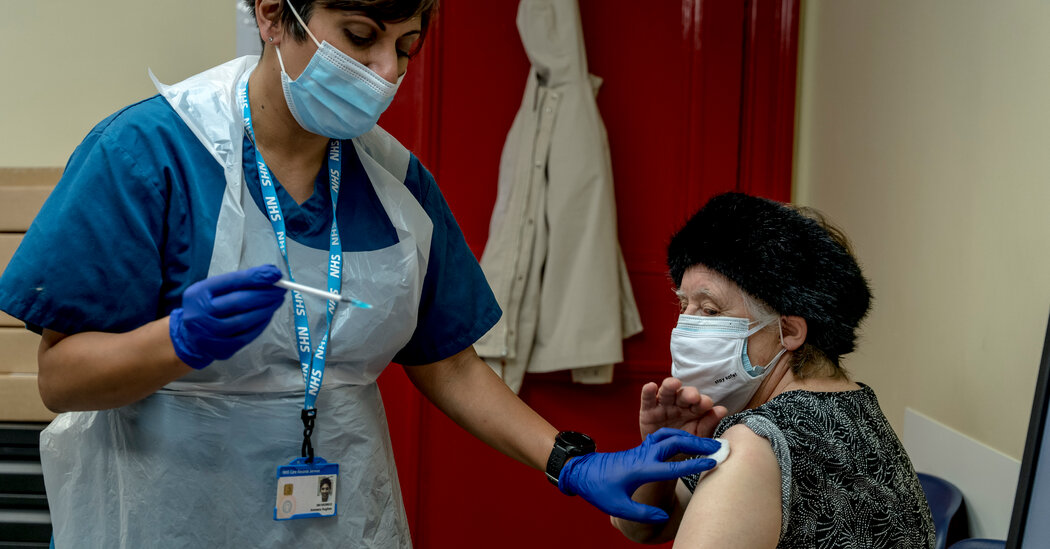
“The vaccination is the only thing we got right,” said Christina Pagel, professor of operational research at University College London.
That doesn’t mean that the rollout was free of tension. With hospital congestion and a contagious variant across the country, the UK has bet on giving more people partial protection from a single dose rather than quickly giving fewer people full protection from two doses.
Doctors whose booster vaccinations were delayed were upset with the approach, accusing the government of making them the subject of a risky new experiment that they fear will make vaccines less effective. Immunologists have raised concerns that a country full of people with only partial immunity could produce vaccine-resistant mutations, while Pfizer said the strategy is not supported by the data gathered in clinical trials.